Désolé, la version française de cette page ne peut être offerte pour le moment.
Golden Rice
“Golden Rice” is the name of a rice that has been genetically engineered (genetically modified or GM) to produce beta carotene, which the body can convert into vitamin A. This beta-carotene gives the rice grains a yellowish colour that inspired its name. It has not been proven as effective but is being widely promoted as an example of how GM can be used to solve important global problems.
In December 2019, the Government of the Philippines approved Golden Rice as safe to eat, following similar approvals by regulators in Canada, the US, and Australia and New Zealand (2017/2018). Its efficacy has not yet been assessed. In July 2021, the Government of the Philippines also approved it for growing, and a limited amount was cultivated and distributed in the country in 2022 and 2023. In April 2024, a court in the Philippines ordered an end to commercial growing of Golden Rice and GM eggplant.
Updates
 June 3, 2024: Farmer network MASIPAG responds to articles in The Guardian on Golden Rice – letter to the editor. “The portrayal of corporate-led solutions as superior not only undermines our efforts but also perpetuates a colonial mindset that we must move beyond.”
June 3, 2024: Farmer network MASIPAG responds to articles in The Guardian on Golden Rice – letter to the editor. “The portrayal of corporate-led solutions as superior not only undermines our efforts but also perpetuates a colonial mindset that we must move beyond.”
May 24, 2024: The Philippines Dodged A Hidden Bullet With Its Ruling Against Golden Rice- Here’s Why, MASIPAG farmers network, Philippines.
April 19, 2024: A court in the Philippines has ordered an end to commercial growing of genetically modified (GM) “Golden Rice” and GM insect-resistant (Bt) eggplant in “an historic win by the Filipino farmers and people” after many years of struggle. Click here to read the press release.
“Today marks a significant victory for the Filipino farmers and people, as well as advocates of food sovereignty worldwide as we prevail in the environmental court battle against Golden Rice and Bt Eggplant. This decision underscores the triumph of farmers and the people in asserting their constitutional right to health and a healthy and balanced ecology against the introduction of unwanted, unnecessary Genetically Modified crops.” – MASIPAG farmer network, Philippines.
April 18, 2023: The Supreme Court in the Philippines has issued a Writ of Kalikasan in a case that seeks to stop the commercial release of genetically modified (GM) “Golden Rice” and GM eggplant. The decision is a response to a petition filed by the farmer group MASIPAG on October12, 2022. “We welcome this move by the Supreme Court in issuing the Writ of Kalikasan on Golden Rice and Bt eggplant and its recognition that these genetically modified crops pose grave danger to our environment and health,” said MASIPAG national coordinator Alfie Pulumbarit. A Writ of Kalikasan is a legal remedy for the protection of the right to a healthy environment, or “a balanced and healthful ecology in accord with the rhythm and harmony of nature,” as stated under Section 16, Article II of the Constitution of the Philippines.
August 8, 2022: Golden Rice and the push for GMOs won’t solve food crisis, it will make it worse, Stop Golden Rice Network.
July 21, 2021: The Philippines’ Bureau of Plant Industry in the Department of Agriculture has issued the biosafety permit for commercial propagation of the genetically engineered Golden Rice.
- Read the July 23 press release from the farmer-scientist organization in the Philippines called MASIPAG: Defend Our Rice! Farmer-scientist group condemns Golden Rice commercial propagation, calls on farmers and consumers to protest
- Hurried Approval Raises Questions, Farida Akhter and Afsar Jafri (Bangladesh) Jul 29,2021
December 20, 2019: The Government of the Philippines has approved Golden Rice for safe human consumption, though its efficacy has not yet been assessed and it is not yet approved for human consumption. “The risks of Golden Rice far outweigh its supposed benefits,” said Dr. Chito Medina, member scientist of the farmers network MASIPAG in the Philippines. “We will be better off improving and diversifying the food crops in the farms and diets of our children to ensure that proper nutrition is achieved.” Read the statement “Farmers and Civil Society Organisations across Asia denounce Philippines’ decision to approve direct use of Golden Rice”.
November 2019: The Canadian Biotechnology Action Network’s new report finds that Golden Rice has low and variable levels of beta-carotene, the beta-carotene degrades significantly during storage and cooking, and has not been adequately tested for bioavailability.
- CBAN Summary (2 pages): “Golden Rice” GM Vitamin A Rice, Update – December 2019.
- CBAN Report: “Golden Rice” GM Vitamin A Rice, November 2019.
March 2018: Health Canada approved the genetically engineered Golden Rice as safe for human consumption, even though the rice is not intended for the Canadian market and has not yet been approved in the intended markets in Asia. Health Canada said, “The efficacy of the GR2E rice in helping vitamin A deficiency in affected populations was not evaluated.”
- March 20, 2018 – Press Release: Health Canada approves GM “Golden Rice” not intended for sale in Canada. For details read below, “Health Canada approved Golden Rice”
The US Food and Drug Administration approved “Golden Rice” as safe in May 2018, and the government agency Food Standards Australia New Zealand (FSANZ) was the first to approve Golden Rice as safe in December 2017.
German non-profit group Testbiotech says, “The risk assessment as performed by FZANZ is not sufficient to demonstrate safety of food derived from GR2.” Read the critique from Testbiotech.
“Stop Golden Rice! Defend our Farmers‘ Rights! remains the resounding call of Asia farmers‘ network against the impending commercialization of Golden Rice in Asia. Waves of protest mobilizations stirs anew in the Philippines and Bangladesh against its commercialization, while debate rages on in Indonesia, India and other Asian countries where Golden Rice is planned for commercial release.” – joint statement from over 30 organizations in the Stop Golden Rice! Network, 2017
Health Canada approves Golden Rice
In 2018, Health Canada approved the genetically engineered Golden Rice as safe for human consumption, even though the rice is not intended for the Canadian market and had not yet been approved in the intended markets in Asia. In the summary of its decision to approve Golden Rice, Health Canada said, “The main intended market for this product is in countries such as Bangladesh and the Philippines where diets are typically low in vitamin A…The International Rice Research Institute has indicated that this product is not intended to be sold in Canada.”
Health Canada said, “The efficacy of the GR2E rice in helping vitamin A deficiency in affected populations was not evaluated.”
- March 20, 2018 – Press Release: Health Canada approves GM “Golden Rice” not intended for sale in Canada
- Read our correspondence with the Minister of Health.
- CBAN Letter to the Editor, The Western Producer, April 12, 2018: Golden Rice assumptions wrong
The 2018 decision by Canadian government regulators (Health Canada) to assess and approve the safety of Golden Rice was not a “humanitarian gesture” ( read CBAN’s Letter to the Editor, The Western Producer, April 12, 2018: Golden Rice assumptions wrong). Health Canada was clear that “the efficacy of the GR2E rice in helping vitamin A deficiency in affected populations was not evaluated” and that “IRRI has indicated that this product is not intended to be sold in Canada.”
So why did Health Canada assess the safety of Golden Rice for Canadian consumption? In its decision, Health Canada noted that “it may be possible that raw commodity or food products derived from GR2E rice may unintentionally enter Canada via imports from countries of production.” In correspondence with CBAN, the department further stated that “Health Canada’s science-based regulatory system for novel foods is internationally recognized and developers often choose to seek authorization in Canada as a first step in their regulatory plan even if they do not plan to sell the product in Canada.” Ultimately, “Health Canada is required by law (i.e., the Food and Drug Regulations) to assess all novel food applications when a notification is filed with the Department.”
“The Canadian government has just waded into a huge global controversy that should be left to Asian farmers and consumers to decide, not Canadian regulators… We need to first give the people and government of the Philippines time to assess and debate Golden Rice, before we weigh in.” – Eric Chaurette, Inter Pares
Background
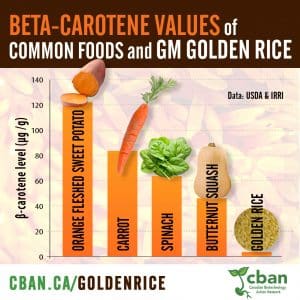 “Golden Rice” GM Vitamin-A Rice, CBAN Report, 2019.
“Golden Rice” GM Vitamin-A Rice, CBAN Report, 2019.
Genetically engineered “Golden rice” is promoted as a solution to the serious health problems resulting from widespread vitamin-A deficiency in the Global South. Golden Rice has been in development for over 20 years and it is still being tested. Golden Rice is still not proven as effective.
Other solutions to vitamin-A deficiency are available, such as delivering vitamin A supplements and fortifying vitamin A in staple foods, and are better targeted and more cost efficient. Furthermore, many conventionally bred plants show a high carotinoid content. Vitamin-A deficiency is a symptom of malnutrition and hunger due to severe poverty.
Vitamin A supplementation and food fortification in the Philippines (began in 1999) has already reduced vitamin A deficiency in preschool age children from 40% in 2003 to 15.2% in 2008 (the World Health Organization considers 15% to be the cutoff for when deficiency is considered a public health problem).
Farmers’ organizations across Asia continue to oppose the release of Golden Rice. Read the joint statement from over 30 organizations in the Stop Golden Rice! Network: “Stop Golden Rice! Defend our Farmers‘ Rights! remains the resounding call of Asia farmers‘ network against the impending commercialization of Golden Rice in Asia. Waves of protest mobilizations stirs anew in the Philippines and Bangladesh against its commercialization, while debate rages on in Indonesia, India and other Asian countries where Golden Rice is planned for commercial release.”
Resources
- CBAN Summary – December 2019: GM “Golden Rice” Vitamin A Rice

- CBAN Report – November 2019: GM “Golden Rice” Vitamin A Rice
- The Philippines has rated ‘Golden Rice’ safe, but farmers may not plant it. Glenn Stone and Dominic Glover, The Conversation. February 2020.
- Biofortified crops or biodiversity? The fight for genuine solutions to malnutrition is on. GRAIN. June 2019.
- Don’t get fooled again! Unmasking two decades of lies about Golden Rice. GRAIN, MASIPAG and Stop Golden Rice! Network. November 2018.
- Asia farmers’ network resounds strong call to Stop Golden Rice! Joint statement by the Stop Golden Rice! Network, August 2017.
- Genetically modified Golden Rice falls short on lifesaving promises: GMO activists not to blame for scientific challenges slowing introduction, study finds. Gerry Everding, Washington University in St Louis, June 2, 2016
- Don’t Eat the Yellow Rice: The Danger of Deploying Vitamin A Golden Rice, Ted Greiner, Independent Science News, July 2016
- “Golden Rice” – GM Vitamin-A Rice, CBAN Factsheet, Updated July 2016
- Golden Rice – a complex tangle of unanswered questions, The Ecologist, February 2014
- Click here for the short update briefing: Status of Golden Rice, January 2014.
- Read “Golden Rice not yet ready”, Letter to the editor, Ontario Farmer, March 2012, by Lucy Sharratt, CBAN Coordinator
- January 2012 report “Golden Lies” from Testbiotech, Germany – click here for a summary and to download the pdf.
- Read the report from Greenpeace “Golden Illusion: The broken promise of Golden Rice”.
For the broader context see CBAN’s GMO Inquiry report “Do we need GM crops to feed the world?”






